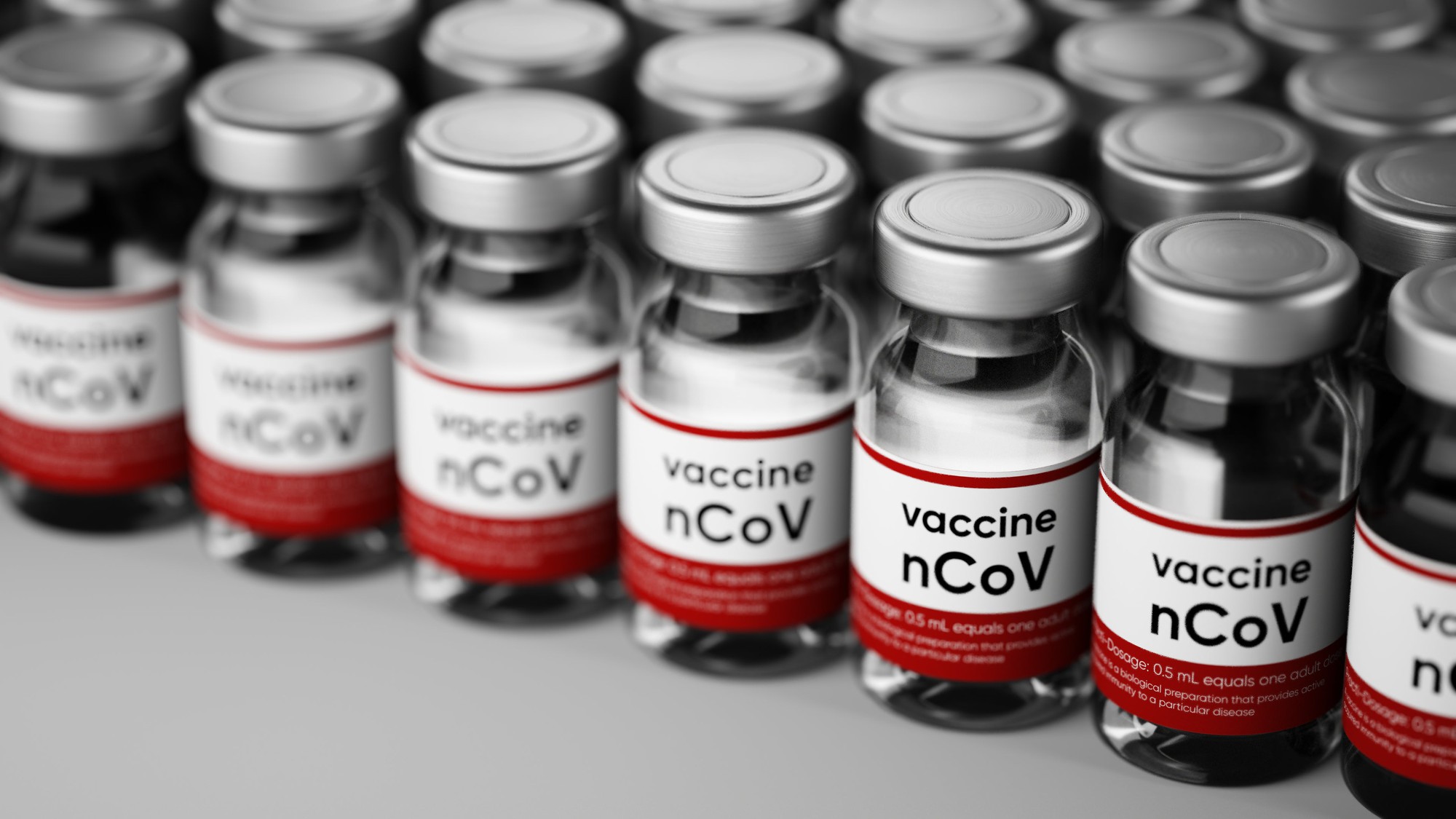
Biotech Moderna (NASDAQ:MRNA) announced an agreement with Swiss drugmaker Lonza Group (OTC:LZAGY) to manufacture up to a billion doses per year of mRNA-1273, Moderna’s vaccine candidate for the novel coronavirus.
The two companies initially plan to establish manufacturing capabilities at Lonza’s facilities in the U.S. and Switzerland to make the vaccine at both locations, with additional production suites to be established across Lonza’s worldwide facilities under the 10-year agreement. The companies expect to produce the first batches of mRNA-1273 at Lonza’s U.S. facility in July 2020. A portion of the funding for the manufacturing operations in the U.S. will come from Moderna’s contract with Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services.
Moderna, which has a platform based on messenger RNA (mRNA) that can develop new vaccine candidates in days, is currently conducting phase 1 trials of mRNA-1273 and has submitted an application to the U.S. Food and Drug Administration (FDA) to initiate phase 2 testing this fall. The company also has the capability to manufacture the vaccine, but the deal with Lonza will increase its capacity by a factor of 10.
Shares of Moderna, already up 135% this year, initially rose another 6% on the news, and the American depositary receipts (ADRs) of Lonza were up 5% in Friday morning trading
By Jim Crumly, The Motley Fool