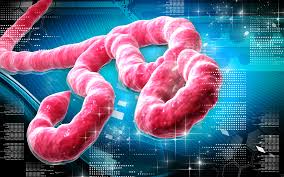
The World Health Organisation (WHO) has okayed the use of blood of patients who recover from Ebola for the treatment of others.
This is one of the cocktail of measures the world health body has approved as the world continues to search for a cure for the Ebola Virus Disease which has claimed 2,105 lives in West Africa, including seven in Nigeria.
WHO also raised global hope with the announcement that Ebola vaccine could be used on the frontline in November when safety data would be ready.
These measures were agreed on at a meeting of a global group of experts to assess the experimental therapies that could contain Ebola.
West Africa is facing the largest Ebola outbreak in history which has so far claimed 1,089 lives in Liberia, 517 Guinea and 491 in Sierra Leone.
The deadly virus was brought to Nigeria by a Liberian-American, Patrick Sawyer, on July 20. Seven deaths had been recorded while eight of the Ebola patients were successfully managed and discharged. Contact tracing has also been underway, with more than 400 contacts being followed in both Lagos and Port Harcourt, while two cases are currently in isolation.
A statement issued by WHO at the weekend said, “150 participants, representing the fields of research and clinical investigation, ethics, legal, regulatory, financing, and data collection, identified several therapeutic and vaccine interventions that should be the focus of priority clinical evaluation at this time.”
The statement said the experts determined that: “There was consensus that the use of whole blood therapies and convalescent blood serums needs to be considered as a matter of priority.
“Safety studies of the 2 most advanced vaccines identified – based on vesicular stomatitis virus (VSV-EBO) and chimpanzee adenovirus (ChAd-EBO) – are being initiated in the United States of America and will be started in Africa and Europe in mid-September. WHO will work with all the relevant stakeholders to accelerate their development and safe use in affected countries. If proven safe, a vaccine could be available in November 2014 for priority use in health-care workers.
“In addition to blood therapies and candidate vaccines, the participants discussed the availability and evidence supporting the use of novel therapeutic drugs, including monoclonal antibodies, RNA-based drugs, and small antiviral molecules. They also considered the potential use of existing drugs approved for other diseases and conditions. Of the novel products discussed, some have shown great promise in monkey models and have been used in a few Ebola patients (although, in too few cases to permit any conclusion about efficacy).”
The endorsement of the blood therapy is based on the medical assumption that people produce antibodies in the blood in an attempt to fight off an Ebola infection. Serum from Ebola survivors should have antibodies that would neutralize infection when given to other patients. Serum is the liquid part of blood collected after blood is allowed to clot separately by gravitational force.
In theory, those antibodies can be transferred from a survivor into a sick patient to give their immune system a boost. However, large scale data on the effectiveness of the therapy is lacking. But studies on the 1995 outbreak of Ebola in Democratic Republic of Congo showed seven out of eight people survived after being given the therapy.
An assistant director general at WHO, Dr. Marie Paule Kieny, said: “We agreed that whole blood therapies may be used to treat Ebola virus and all efforts must be invested to help infected countries to use them.
“There is a real opportunity that a blood-derived product can be used now and this can be very effective in terms of treating patients.”
She said that it was the one positive aspect of so many people being infected.
“There are also many people now who have survived and are doing well. They can provide blood to treat the other people who are sick.”
There is no clinically proven drug or vaccine to treat Ebola, but many are in the experimental stage.
Around 150 experts spent the last two days investigating how to fast-track promising experimental drugs to make them available in West Africa as soon as possible.
Ebola vaccine trials started in the US last week and will be extended to centres in the UK, Mali and Gambia in the coming weeks.
The first person to take part in a vaccine trial was a 39-year-old in the US
The WHO said safety data would be ready by November 2014 and, if it proved safe, would be used in West Africa immediately.
Healthcare workers and other frontline staff would be prioritised for vaccination, the WHO said.
Experimental drugs – such as ZMapp, which has been used in seven patients including a British volunteer nurse – were also assessed.
However, the supplies of all the experimental drugs are very limited, if not exhausted.
The WHO said efforts were underway to increase production, but it would take several months.
Dr Jesse Goodman, from Georgetown University Medical Center in the US, took part in the meeting.
He said: “This is a unique opportunity to identify what new treatments and vaccines can really help people and then potentially accelerate their use.
“We don’t want to end up after this outbreak not knowing how best to prevent or treat the next one.”
Yet WHO warned that all the talk of experimental therapies must not detract from the proven methods of infection control which have defeated all previous outbreaks.