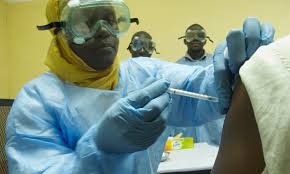
As Nigeria eagerly awaits the Ebola Virus Disease (EVD)-free status declaration by the World Health Organisation (WHO) on Monday, a group of world researchers have raised some salient issues surrounding the controlled trials for the Ebola vaccine.
In a lead presentation titled: “Randomised controlled trials for Ebola: Practical and ethical issues” and published in The Lancet, on 13 October, the researchers made reference to the WHO’s approval for trial use of an investigational drug and vaccine on Ebola patients, noting that although the question of whether unproved treatments should be offered at all is now settled, the question of how they should be deployed and tested is not.
The team of researchers include Clement Adebamowo of the National Health Research Ethics Committee, Abuja, Nigeria; Oumou Bah-Sow of Hôpital National Ignace Deen, Conakry, Guinea; Fred Binka of University of Health and Allied Sciences, Ho, Ghana; Roberto Bruzzone of Hong Kong University-Pasteur Research Pole, School of Public Health, University of Hong Kong, Hong Kong, China and Arthur Caplan of New York University Langone Medical Centre, New York, USA.
Others include Jean-François Delfraissy of Institut de Microbiologie et Maladies Infectieuses and INSERM, Paris, France; David Heymann of Centre on Global Health Security, Chatham House, London, UK; Peter Horby of University of Oxford, Oxford, UK; Pontiano Kaleebu of Medical Research Council, Uganda Virus Research Institute, Entebbe, Uganda; Jean-Jacques Muyembe Tamfum of Institut National de Recherche Biomedicale, Kinshasa, DR Congo; Piero Olliaro of WHO, Geneva, Switzerland; Peter Piot of London School of Hygiene & Tropical Medicine, London, UK; Abdul Tejan-Cole of The Open Society Initiative for West Africa, Dakar, Senegal; Oyewale Tomori of the Nigerian Academy of Science, Lagos; Aissatou Toure of Institut Pasteur Dakar, Dakar, Senegal; Els Torreele of Open Society Foundations, New York, USA and John Whitehead of Lancaster University, Lancaster, UK.
According to the researchers, the acute nature of the EVD outbreak in West Africa had made the WHO to declare that it is ethical to offer interventions with potential benefits but unknown efficacy and side-effect albeit every effort should be made to evaluate benefits and risks and share all data generated.
They observed that the need for drugs and vaccines was urgent then. But with cases now rising exponentially and health systems overwhelmed, it is even greater today.
“Vaccine safety trials are underway in the USA and the UK, and poised to roll out to Africa soon. But treatments for those with infection are required too,”, the research team noted.
“Besides playing a direct part in containing the epidemic, interventions that could improve outcomes for the sick would help to rebuild the confidence of affected communities in health services, a critical step if Ebola is to be overcome,” they said.
A fast-track initiative for evaluating investigational drugs was launched in September 2014.
“Still at issue is whether such treatments should be made available only in the context of randomised controlled trials (RCTs) in which patients receive either a new intervention and conventional care, or conventional care alone or with a placebo.
“Advocates of this RCT approach state that as this experimental design will create the most robust evidence for the future, and is what regulators are used to, it is the only approach that should be considered. We disagree.
“While we concur that RCTs provide robust evidence, and support their use where this is ethical and practical, we do not believe that either consideration is likely to be satisfied in the context of this epidemic