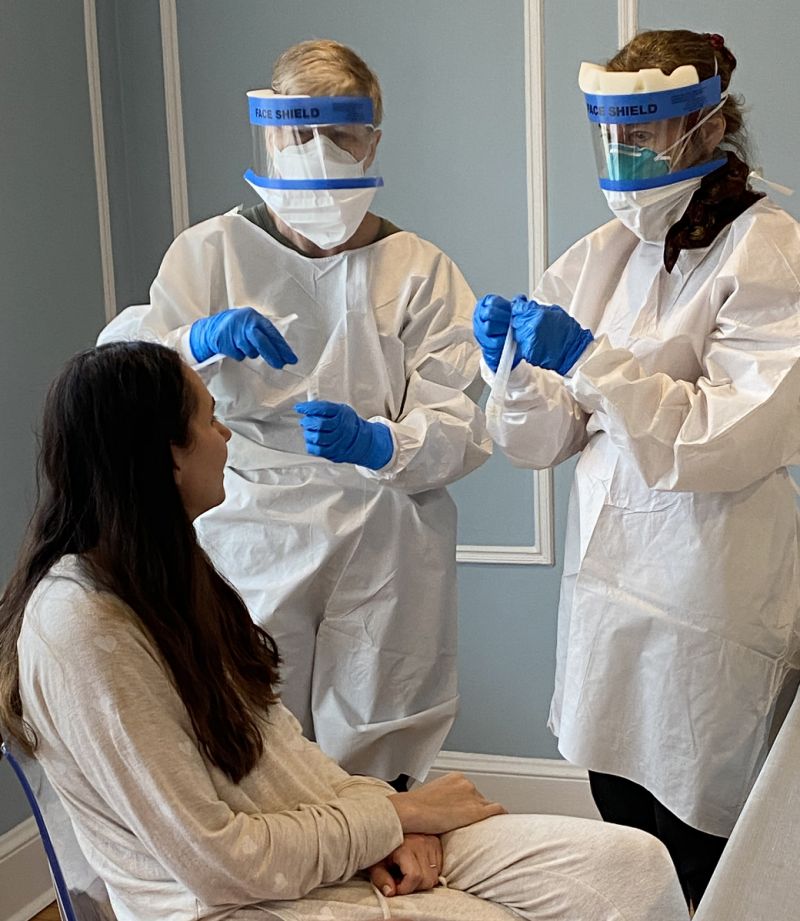
As patients with COVID-19 continue pouring into emergency departments and intensive care units across the nation, an old treatment that has been adapted for a new disease is being tested in New York.
In the past few days, the Mount Sinai Hospital System has injected more than 20 very sick coronavirus patients with a “convalescent serum” based on the blood plasma of people who have recovered from the disease.
One of the first recovered patients to donate, Danny Riemer, 37, of New Rochelle, New York, said he and his wife feel “blessed” that they are now healthy and can volunteer their plasma to help others. “And despite the fact that we did have the virus, our thoughts are really with others, the people who are still fighting the virus, the people who have had much more serious cases than us,” he said.
Full coverage of the coronavirus outbreak
Dr. Jeffrey Jhang, medical director of clinical laboratories and transfusion services for the Mount Sinai Health System, said: “We have so many patients who are sick. We are crossing our fingers that this will be a game changer and really accelerate the recovery of these patients.”
As first reported in a series of stories by NBCNews.com, Dr. Arturo Casadevall of the Bloomberg School of Public Health at Johns Hopkins University in Baltimore has spearheaded a nationwide effort to develop a plasma treatment.
The National COVID-19 Convalescent Plasma Project is a consortium of more than 40 of the nation’s top health institutions across 22 states looking to collect plasma from recovered COVID-19 patients to assist in treating those currently ill with the disease or to perhaps prevent others, like front-line medical workers, from getting the virus at all.
Convalescent plasma therapy, which involves collecting antibodies from the blood of recovered patients, is nothing new. It was used to treat the 1918 and 1957 flu epidemics, as well as SARS, H1N1 and Ebola and, most recently, some COVID-19 patients in China.
But in recent weeks, Dr. Florian Krammer, a microbiologist, and his team at Mount Sinai’s Icahn School of Medicine have made a breakthrough in COVID-19 antibody testing.
Jhang said: “While other tests measure whether the antibody is there or not, our assay can also measure how much of the antibody is there. And that’s important, because we can identify those donors with a high amount of antibody who would most likely benefit the patients receiving this plasma.”
The more antibodies, it’s thought, the greater the boost to the treatment’s efficacy.
Jhang said that the hospital is using an Emergency Use Authorization from the Food and Drug Administration to conduct the tests but that the agency has discussed issuing a formal protocol so more hospitals can use the test.
On Friday, the FDA issued an updated set of guidelines reminding health care providers that more clinical trials must take place before “routinely administering” convalescent plasma to COVID-19 patients can begin.
When it comes to executing the practice on a wide scale, Jhang said, part of the challenge lies in waiting for enough patients to fully recover and develop immune responses. Then they must be screened. Each donor provides the equivalent of four doses of plasma. At Mount Sinai, two doses are being given to the sickest patients. In the past few days, Mount Sinai has given more than 20 very sick patients the plasma of those who recovered.
By Conor Ferguson and Cynthia McFadden and Didi Martinez, NBC News