By Krishna N. Das, Reuters
NEW DELHI (Reuters) – India on Sunday granted emergency approval to Bharat Biotech’s COVAXIN but faced questions from industry experts and opposition lawmakers after taking the step without publishing efficacy data for the homegrown coronavirus vaccine.
The news, announced by the drugs controller general of India (DCGI), was hailed by Prime Minister Narendra Modi and his ministers as a success in the country’s self-reliance push.
The government also approved the use of a vaccine developed by AstraZeneca and Oxford University which will be the lead vaccine in India’s immunisation programme.
COVAXIN was jointly developed with a government institute and means India joins a small list of countries to have approved their own coronavirus vaccines.
Bharat Biotech has partnered with drug developer Ocugen Inc to co-develop it for the U.S. market, and Brazil has signed non-binding letters of intent to buy the shot.
The company has said it is in discussions with more than 10 countries about COVAXIN.
“Our goal is to provide global access to populations that need it the most,” Bharat Biotech Chairman Krishna Ella said in a statement.
“COVAXIN has generated excellent safety data with robust immune responses to multiple viral proteins that persist.”
Neither the company nor India’s Central Drugs Standards Control Organisation would reveal its efficacy results. A source with knowledge of the matter told Reuters its effectiveness could be more than 60% with two doses.
China also did not publish detailed efficacy data for a vaccine it authorised on Thursday but its developer has shared interim data.
“On what basis was this approval given when Bharat Biotech has NOT shown enough data proving safety & efficacy?” transparency activist Saket Gokhale asked on Twitter.
Gokhale has filed a request under India’s right-to-information law asking the government for safety and other data for the two vaccines approved on Sunday.
Serum Institute of India (SII), the local maker of the AstraZeneca shot that will be branded COVISHIELD in the country, also took a dig at the Bharat Biotech vaccine.
“Until we get the efficacy result, how do we know that anything works?” SII Chief Executive Adar Poonawalla told Reuters.
A government scientist said in November COVAXIN might be approved in February or March. It is now likely to be rolled out much earlier following the approval on Sunday.
Opposition lawmakers and former ministers on Sunday questioned the lack of transparency in approving it.
“Approval was premature and could be dangerous,” said opposition lawmaker and former minister Shashi Tharoor.
“Its use should be avoided till full trials are over. India can start with the AstraZeneca vaccine in the meantime.”
Vardhan urged Indians to trust that “stringent protocols” had been followed for the two approved vaccines.
In the largest such trial in India, Bharat Biotech said it had recruited 23,000 volunteers out of a target of 26,000 for an ongoing Phase III trial of COVAXIN which began in November.
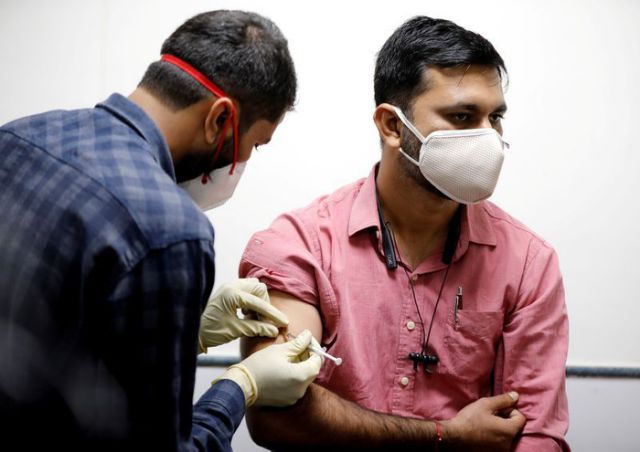
FILE PHOTO: A medic administers COVAXIN, an Indian government-backed experimental COVID-19 vaccine, to a health worker during its trials, in Ahmedabad
(Additional reporting by Aftab Ahmed and Nigam Prusty; editing by Jason Neely and Alexandra Hudson)