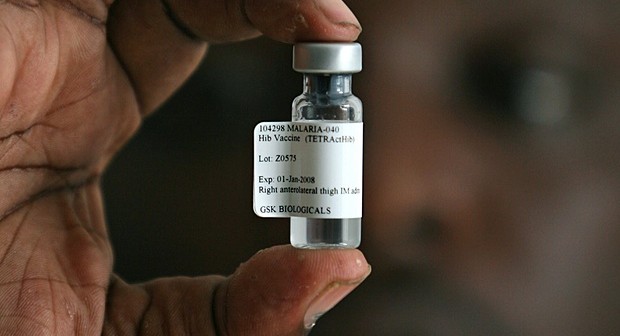
Nigerian scientists led by the President of Nigerian Academy of Science (NAS), Prof. Oyewale Tomori, and National Coordinator, National Malaria Elimination Programme (NMEP), Dr. Nnenna Ezeigwe, have hailed the world’s first malaria vaccine, which represents a major step toward prevention of a disease that kills more than half a million people worldwide every year – most of whom are children in Africa.
They, however, urged caution in the celebration of the breakthrough , saying the vaccine has to be approved by the National Agency for Food Drug Administration and Control (NAFDAC) before use in Nigeria.
The scientists said the new vaccine would at best be an addition to all the other interventions that are already on- going in the malaria control efforts in the country and will definitely not replace other preventive or curative strategies.
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP), after assessing the quality, safety and efficacy of the vaccine – called RTS,S (brand name Mosquirix) – on Friday July 24, 2015, announced it should be used for immunization of children in Africa aged six weeks to 17 months, alongside other protective measures against malaria – such as insecticides and bed nets.
The CHMP recommendation is the first step toward RTS,S becoming the first licensed vaccine for malaria. Later this year, independent advisory groups from the World Health Organization (WHO) will review evidence for the vaccine and decide whether to recommend its use.
Tomori told The Guardian yesterday: “The vaccine was tested in many African countries. I think there was a trial in Nigeria, but am not sure. It only offers partial protection, it is an addition to the armoury against malaria.
“It calls for celebration. Any little progress, after so many years deserve celebration, but not in excess, because no one is really sure of the full impact when introduced for routine vaccination.
“We must take advantage of the vaccine, but remember is not a replacement of other measures against malaria – impregnated nets, vector control, use of anti-malarial drugs and simply keeping our environment clean and not a playing field for breeding mosquitoes.”
Ezeigwe said: “For Nigeria this is good news. The development of the vaccine has been on for about 30 years. Many candidate vaccines could not make it this far. So since about two years ago when it became clear that this particular one (RTS,S) has a lot of promise we have waited with cautious optimism that it will scale all the hurdles of clinical trials. The development makes us a bit more confident in the malaria fight because when approved by the WHO it will be an addition to the arsenal for the fight against malaria.
“Well for celebration I would rather reserve it for when we have a demonstrable evidence that malaria is no more a significant public health concern. Although we have just reached a critical landmark in the quest for this vaccine, there are still a couple of steps more before it can be deployed as an intervention. With this certification by the European Medicines Agency, the next step is for WHO to make a policy statement to recommend it for use. It will now be the responsibility of individual countries to register it as a mark of approval for it to be used locally.
“In our own case NAFDAC has to approve it before the Federal Ministry of Health can take a policy stand on it.
“The new vaccine will at best be an addition to all the other interventions that are already on going in the malaria control efforts in the country. It will definitely not replace other preventive or curative strategies. The target for the vaccine are children under the age of five and even at that, it is more effective in children between five and 17 months where about 50 per cent of cases can be prevented.
“Deploying the vaccine in this group of children will boost the fight against malaria because children constitute the most vulnerable group for malaria but that alone will definitely not enable us to win the war. Universal coverage with preventive, diagnostic and preventive measures are required for meaningful progress towards elimination.
“Yes the clinical trial was conducted in seven African countries and involved research centers in these places, including Burkina-Faso, Gabon, Ghana, Kenya, Malawi, Mozambique and Tanzania. Part of the required tests was also done in Nigeria at some point. Two groups of 16,000 children aged between six and 12 weeks and five and 17 months respectively were tested in all. Having been tested in Africa, we are more confident that this vaccine will be of great benefit to us in Nigeria.” The Guardian